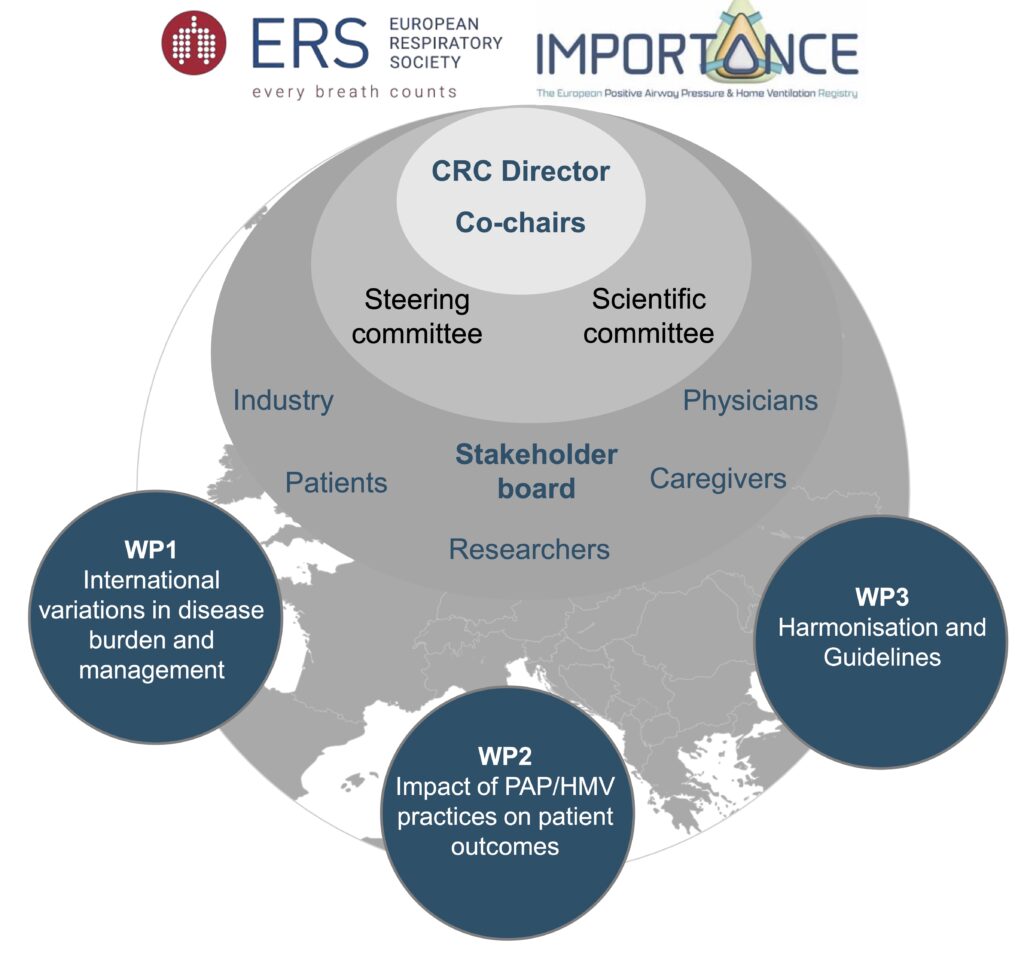
Work Package 1: International variations in disease burden and management
Work Package 1: International variations in disease burden and management
We are surveying patients, clinicians and home care providers to gather insights on care delivery and outcomes, and identifying existing local and national registries. Following legal requirements for data regulation, these data will be merged to provide retrospective insights into care delivery.