The SHARP FAP enables analysis of data from individual registries, without patient data leaving the country of origin, thus respecting data privacy rules. The SHARP FAP uses a distributed model, in which R-code is submitted to the host institution and summary statistics are returned. These summary statistics are combined from registries across Europe via formal meta-analysis, resulting in powerful and clinically meaningful results.
SHARP CRC engaged with ITTM (Information Technology for Translational Medicine S.A.) for the set-up of a Federated Analysis Platform and harmonised data model based on the Observational Medical Outcomes Partnership (OMOP) common data model (CDM).
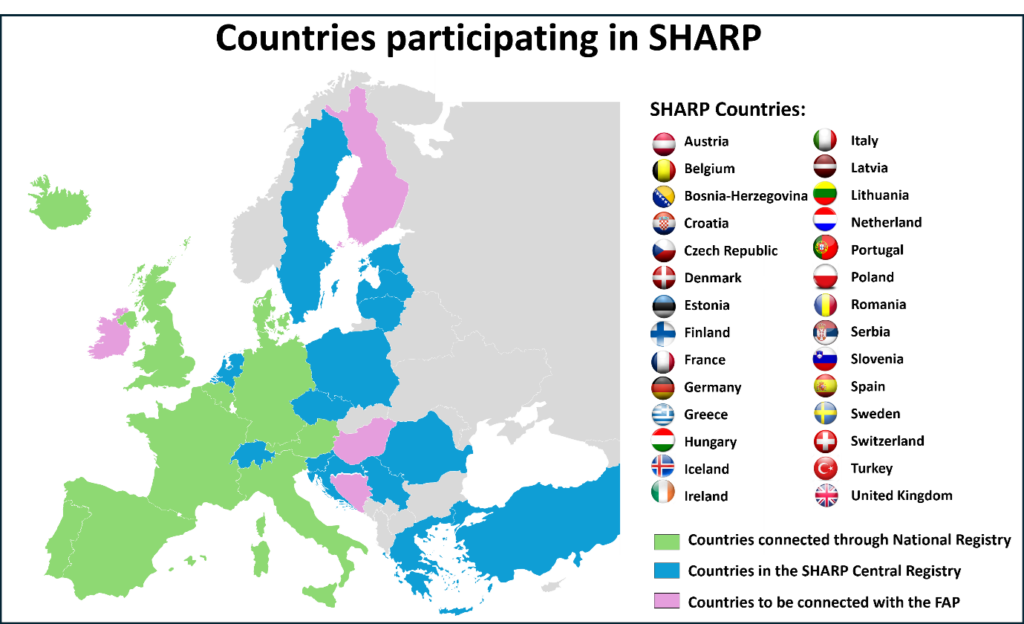
So far 24 European countries have engaged in the SHARP FAP – providing a platform for analysis of more than 14,000 patients with severe asthma.
SHARP Central (started in 2018) is a novel registry of patients with severe asthma in European countries founded by the CRC SHARP. It helped countries that did not yet have their own national severe asthma registry to save time and funding and to quickly become active.
The SHARP Central registry has the following properties:
So far 14 countries are part of SHARP Central: Croatia, Czech Republic, Estonia, Greece, Hungary, Latvia, Lithuania, The Netherlands, Poland, Romania, Serbia, Slovenia, Sweden and Turkey. 3 countries are in process of integrating SHARP Central: Bosnia and Herzegovina, Ireland and Switzerland
The current number of patients is 4,311.
The goal it to have access to an overview of the data/variables collected in the different registry and among them which are accessible (comprised in/aligned with the SHARP CDM) for research.
SHARP members can connect to the “data catalogue” and ask research questions based on the accessible/available variables.
There are 3 tables:
Only members of the SHARP CRC have access to the SHARP Data catalogue.
To become a SHARP member and access the information please contact the SHARP project manager.
You can provide your feedback by filling in the questionnaire: